Inspire Sleep Apnea Implant Mri Safety
Inspire uas is used in adult patients 22 years of age and older who have been confirmed to fail or cannot tolerate positive airway pressure pap treatments such as continuous positive.

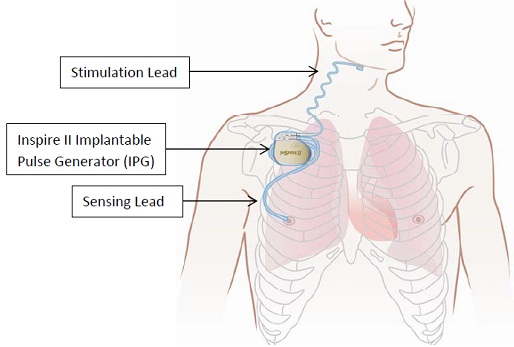
Inspire sleep apnea implant mri safety. Patients who have any component of the inspire system implanted should not undergo mri. Untreated obstructive sleep apnea osa has a negative impact on sleep and the bodys ability to recover during sleep. However the design of the newest model the inspire 3028 includes conditional mri labeling that will allow patients safe access to mri scans.
If so they are eligible for mri head and extremity scans provided specific guidelines in the manual are followed. There is a yellow mr triangle on the back of the patient id card if your patient has the inspire generator model 3028. To find out what type of inspire model you have please contact your doctor or look at your patient id card.
If your patient has the inspire generator model 3024 they are not eligible for mri scans. Important safety information inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients with an apnea hypopnea index ahi between 15 and 65. Sleep remote use when.
Inspire therapy has not been tested in people with a bmi greater than 32. As a result osa patients may experience a high degree of daytime sleepiness. Inspire upper airway stimulation uas is used to treat a subset of patients with moderate to severe obstructive sleep apnea osa apnea hypopnea index ahi of greater than or equal to 15 and less than or equal to 65.
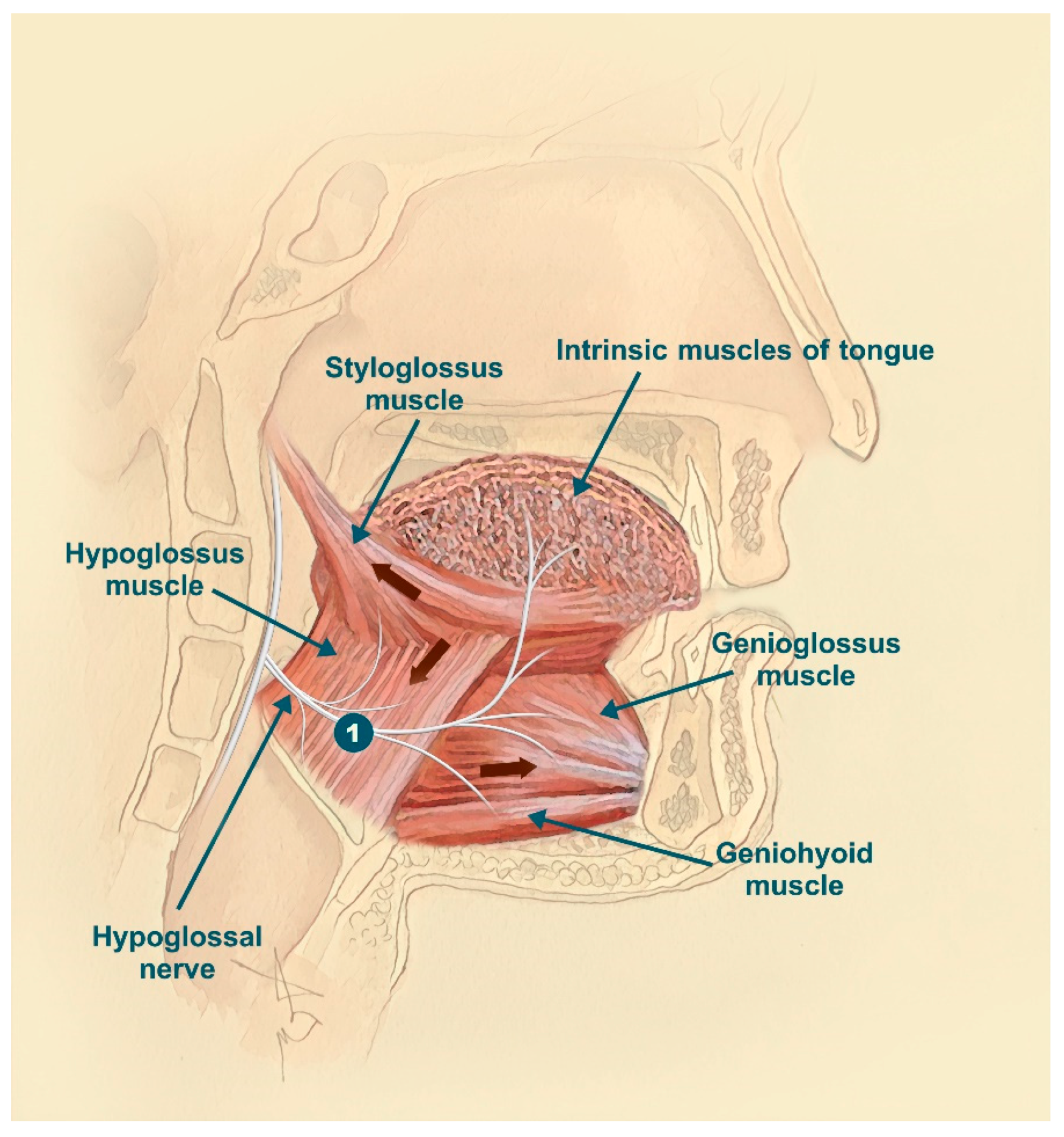
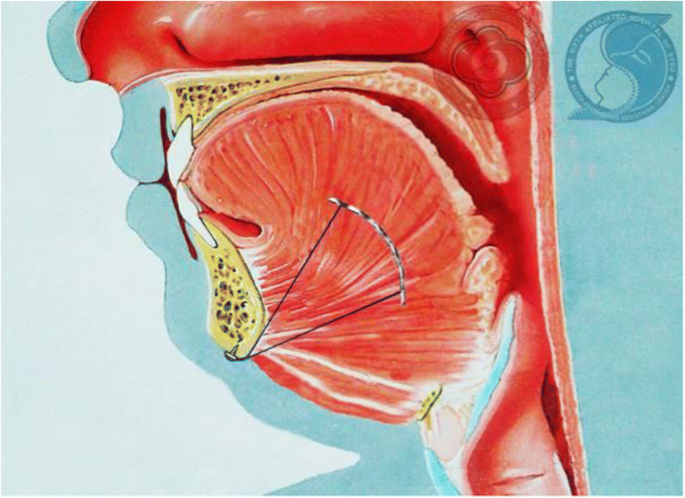
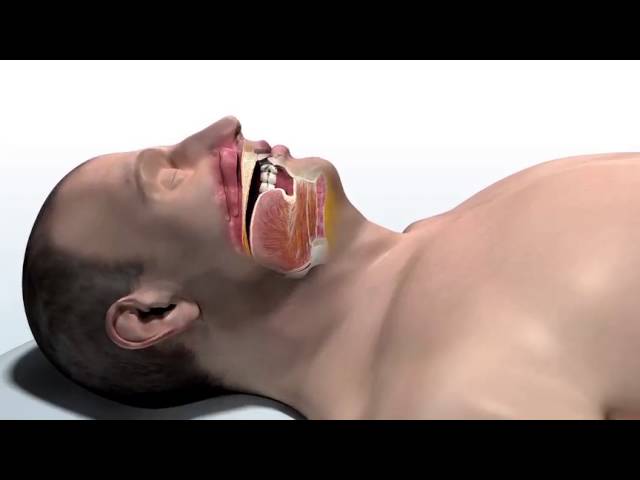
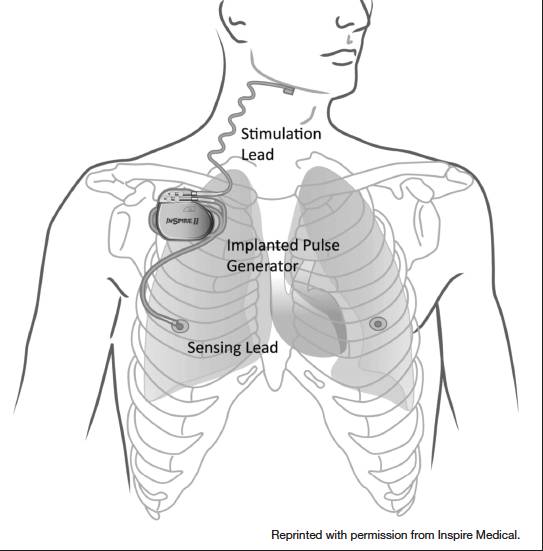
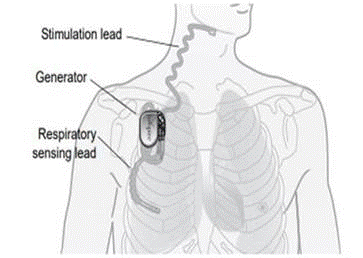
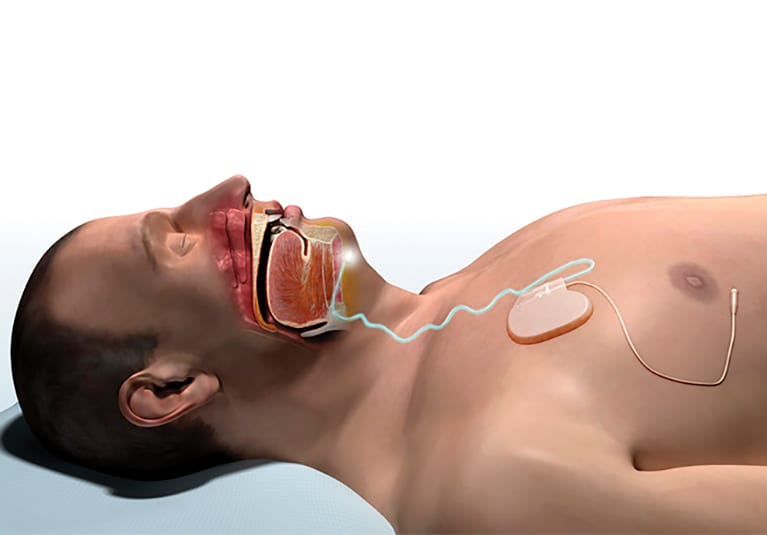
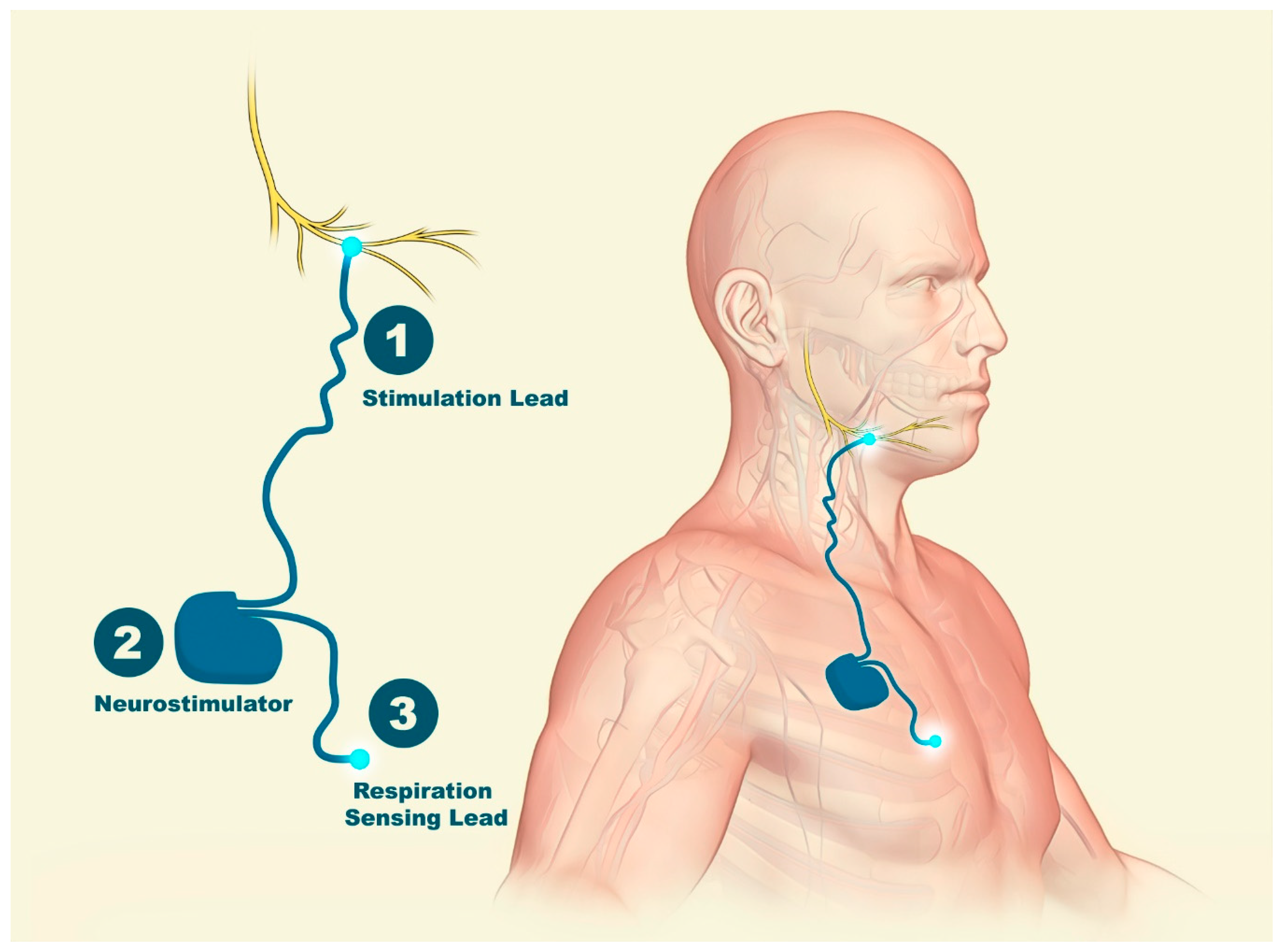
The inspire stimulator is a surgically implanted device that activates the hypoglossal nerve to tighten the muscles of the tongue and upper airway during sleep improving airflow and reducing sleep apnea. If you have an inspire model 3028 you are eligible for mri head and extremity scans provided specific guidelines are followed. It should be used after you have tried positive airway pressure treatments and they have not worked or you could not tolerate them.
It is approved for use by those with. There is a yellow mr triangle on the back of the patient id card if you have the inspire model 3028. Moderate to severe obstructive sleep apnea with apnea hypopnea index ahi greater than15.
Inspire Medical Systems Announces Fda Approval Of Inspire 3028 Neurostimulator For The Treatment Of Obstructive Sleep Apnea Nyse Insp
www.globenewswire.com
Shock To The System An Examination Of Neurostim Technologies Medical Product Outsourcing
www.mpo-mag.com

Inspire Sleep Apnea Implant Treatment Offers Alternative To The Cpap Machine Apnea Today
www.apnea.today





:max_bytes(150000):strip_icc()/man-snoring-because-of-apnea-lying-in-the-bed-833441708-37940fc38f324efead237023b51c8094.jpg)











































/GettyImages-102284500-5c19b889c9e77c0001f1ee9c.jpg)

/cdn.vox-cdn.com/uploads/chorus_asset/file/19533967/AdobeStock_111873047.jpeg)





/cdn.vox-cdn.com/uploads/chorus_asset/file/19533957/Adobeillustration.jpg)